Jenn Hoskins
14th November, 2025

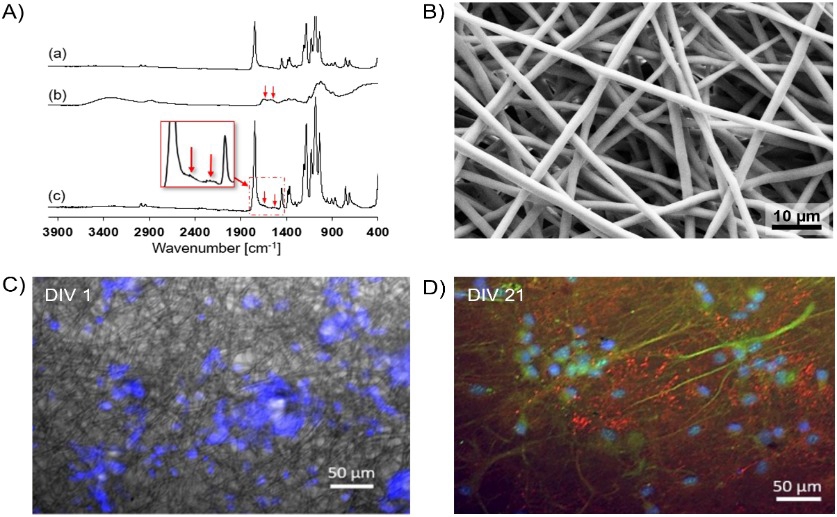
Scanning electron microscopy and immunofluorescence imaging demonstrate successful chitosan coating of electrospun PLA fibers and subsequent adhesion and synaptic differentiation of Sprague-Dawley rat neurons cultured on the scaffold.
Key Findings
- Researchers at the University of Genoa studied how nutrients reach cells within 3D scaffolds made of chitosan and PLA, crucial for growing neural tissues
- Computer modelling showed nutrient delivery is affected by scaffold structure, with PLA offering more predictable transport but both types creating nutrient gradients
- The study highlights optimizing scaffold design—considering porosity and cell density—is vital for ensuring even nutrient distribution and healthy cell growth in lab-grown tissues
The study[1] focused on two types of scaffolds: one made of chitosan microbeads (tiny spheres) and another made of PLA fibers (a type of plastic) coated in chitosan. Both materials are biocompatible, meaning they don’t harm cells, and are often used in tissue engineering. The goal was to understand how nutrients, specifically glucose, move through these scaffolds and are used by the cells growing within them. This understanding is crucial for designing scaffolds that support healthy cell growth and accurately predict how tissues will behave.
To achieve this, the researchers used a combination of theoretical approaches and computer modelling. First, they used “homogenization theory” – a mathematical technique that simplifies complex structures by treating them as an average material. This allowed them to estimate how easily nutrients could move through each scaffold type. Then, they built a detailed computer model using COMSOL, a software package for simulating physical processes. This model accounted for both the diffusion of nutrients and their consumption by the growing cells. The model incorporated glial and neuronal cells, the two main cell types found in the nervous system.
The modelling revealed that nutrient diffusion is indeed affected by the scaffold structure. The PLA fiber scaffold, due to its more organized structure, showed more predictable nutrient transport compared to the chitosan microbead scaffold. However, both scaffolds exhibited nutrient gradients, meaning that cells closer to the edge of the scaffold received more nutrients than those in the center. This is consistent with findings from other studies[2] which demonstrate that oxygen distribution within 3D cultures is often uneven, leading to hypoxia (low oxygen) in the core of larger aggregates.
This research builds on the growing understanding that simply growing cells in 3D isn’t enough; the structure of that 3D environment is critical[3][4]. The study highlights the importance of considering how nutrients are delivered to cells when designing scaffolds for tissue engineering. By using computer modelling, the researchers were able to predict how different scaffold properties would affect nutrient availability, offering a way to optimize scaffold design before conducting expensive and time-consuming experiments.
The findings have implications for a range of applications, including developing therapies for spinal cord injuries. As demonstrated by research into spinal cord bridges[5], creating a supportive environment for cell growth and nerve regeneration is crucial for successful treatment. Understanding how to optimize nutrient delivery within these scaffolds could significantly improve their effectiveness. The work done by the University of Genoa and collaborating institutions provides a valuable tool for designing more effective 3D tissue engineering scaffolds and ultimately, for creating functional tissues in the lab.
References
Main Study
1) Computational analysis of 3D biopolymeric porous scaffolds for the in vitro development of neural networks
Published 12th November, 2025
https://doi.org/10.1371/journal.pone.0335432
Related Studies
Related Articles