Study design
The study adopted a cross-sectional research design.
Study location
This study was conducted in Makurdi, Benue State. Makurdi is the state capital of Benue state, located in central Nigeria (North Central region), and part of the Middle Belt region of central Nigeria. Makurdi has two public tertiary hospitals (Federal Medical Centre, Makurdi, and Benue State University Teaching Hospital, Makurdi) and one secondary hospital (General Hospital, Makurdi, Benue State).
Sample size determination
The minimum sample size for this study was calculated using the Araoye22. The formula is structured to determine the minimum number of participants needed to estimate a population proportion (in this case, the prevalence of diabetes mellitus) with a certain level of confidence and precision.
$$\:N=\frac{{Z}^{2}pq}{{d}^{2}}$$
Where:
N = minimum sample size.
Z = A constant of 95% confidence level = 1.96.
p = prevalence of diabetes mellitus in Norther Central Nigeria (4.3%) = 0.04323 (WHO, 2022).
q = 1-p (1-0.038). The proportion of the population that does not have diabetes.
d = desired precision 1.9% = 0.019. This is the acceptable difference between the sample proportion and the true population proportion.
Therefore:
$$N = 1.96^{2} \times 0.043 \times \frac{{\left( {1 – 0.043} \right)}}{{0.019^{2} }}$$
The minimum sample size was 438. It was rounded up to 571 to cater for attrition.
Sampling technique
The population size of diabetes patients in the three hospitals is 934. The number of respondents selected from each hospital was proportionate to the population size. Federal Medical Centre, Markurdi has a population of 564, Benue State University Teaching Hospital (BSUTH) has a population of 204 and General Hospital, Makurdi (GHM) has a population of 166 diabetic patients receiving treatment at these hospitals during the period of data collection. Respondents who were willing to participate in the study were selected using a simple random sampling technique. Patients who attended diabetes clinic on the day of data collection were asked to pick a ballot of 0 and 1. Only respondents who picked the ballot with 1 participated in the study. A total of 571 respondents were selected from the three hospitals. Three hundred and forty-five diabetic patients were selected from FMCM, 123 diabetic patients from BSUTH, and 101 diabetic patients from GHM.
Eligibility criteria
Eligible patients for this study were only male and female adults 18 years of age and above, out-patients, diagnosed with diabetes mellitus and receiving treatments in the selected hospitals for at least 6 months, not suffering from debilitating or life-threatening complications, were cognitively intact (as determined by their medical records), and willing to participate in the study. Respondent who did not give their consent, and had hearing problems were excluded from the study.
Ethical consideration
Ethical approval was obtained from the Federal Medical Centre, Makurdi, Benue State (FMH/FMC/HREC/108/VL. 1), BSUTH/MKD/HREC/2022/169) and Benue State Hospital Management Board (HMB/OFF/215/VOL. II/535). All methods were performed in accordance with the relevant guidelines and regulations.
Informed consent
Informed consent was obtained from all subjects and/or their legal guardian(s) before data collection. Only respondents who consented participated in the study. Data collected were used strictly for research purposes.
Respondents’ personal and clinical data
A semi-structured interviewer-administered questionnaire was used to obtain information on Patients’ characteristics. Respondents’ clinical characteristics were extracted from their case notes.
Biochemical data
The blood pressure (BP) of respondents was measured using a mercury sphygmomanometer KENZ model 605P on the left arm in a sitting position. It was measured twice at five-minute intervals, and the average was determined. The random blood glucose level of respondents was assessed using Accu-Check Active glucometer, model no: HM100005. The glycated hemoglobin was measured using the Tosoh G7 HPLC analyzer which was calibrated using the diabetes control and complications trial (DCCT) standard with a coefficient of variations < 2.5%. Blood pressure (systolic/diastolic pressure) was classified as normal (< 120/<80mmHg), pre-hypertension (120–139/80-89mmHg), hypertension stage 1 (140–159 / 90-99mmHg) and hypertension stage 2 (> 160/>100mmHg) according to the Joint National Committee on Prevention, Detection, Evaluation, and Treatment24, fasting blood glucose was classified as “Normal” (70-99 mg/dl), “Prediabetes” (100-125 mg/dl) and “Diabetes” (126 mg/dl or above)25 while the glycated hemoglobin was classified as normal (< 5.7%), pre-diabetes (5.7–6.4%) and diabetes (≥ 6.5%)26.
Respondents’ lifestyle behaviours
The lifestyle behaviours assessed were smoking patterns, alcohol drinking patterns, physical activity levels, dietary habits, overweight/gross obesity, and abdominal obesity.
Smoking and alcohol drinking status
The smoking and alcohol drinking patterns were assessed using a semi-structured interviewer-administered questionnaire.
Physical activity level
The physical activity of the patients was assessed using a 27-item International Physical Activity Questionnaire. The questionnaire has domains on job-related; transportation; housework, house maintenance, and caring for the family; and recreation, sport, and leisure-time physical activity. The questionnaire was scored based on a continuous variable score expressed as Metabolic Equivalent (MET) in minutes per week. MET level* minutes of activity* events per week. MET levels used were 3.3 METs (Walking), 4 METs (Moderate activity), and 8 METs (Vigorous activity). Total MET-min/week = (Walk METs*min*days) + (Mod METs*min*days) + Vig METs*min*days) and categorized as inactive (< 600 MET-min/week), minimally active (600–2999 MET-min/week) and heap active (> 3000 MET-min/week)27.
Dietary habit
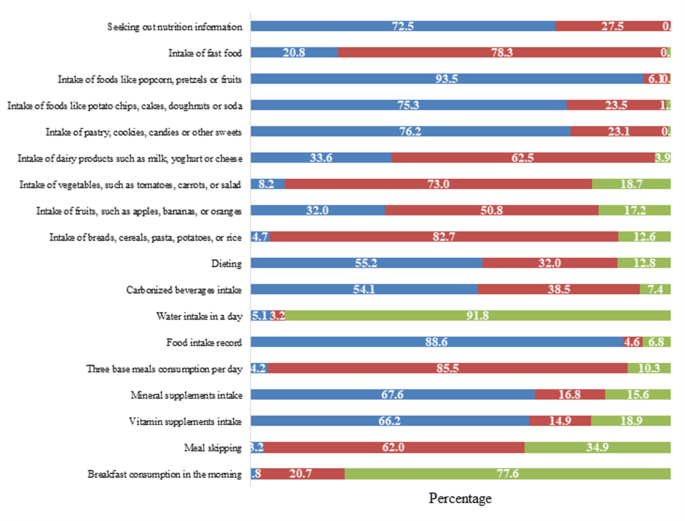
Respondents’ dietary habit was assessed using a 4-point scale 18-item validated questionnaire with a reliability coefficient of 0.6628. Respondents were asked to identify how often a particular food item is eaten. These questions included how often the subject consumed foods from each portion of the food pyramid, as well as the consumption of beverages, and vitamin and mineral supplements. Food intake, dieting, and skipping meals were also examined. For this section, answers ranged from always (4) to never (1). Question numbers 2–4, 8–9, 14–15, and 17 were reversed scored. This means that if the subject answered the question with a 1, then he/she was awarded 4 points. All other questions were scored according to their value. Items were scored by summing up responses. The obtainable score ranged from 18 to 72. The higher the scores, the better the eating habits. The percentage from the highest score obtainable was calculated and classified as “excellent” (76–100%), “good” (51–75%), “fair” (26–50%) and “poor” (0–25%).
Overweight/obesity and abdominal obesity
Respondents’ body weight, height, and waist circumference were measured using standard procedures29. Body mass index was calculated and classified as > 25.0 kg/m2 and ≥ 25.0 kg/m2 using the WHO classification29. Waist circumference was used to assess abdominal obesity (i.e. accumulation of fat around the abdominal region). Waist circumference was classified as “Normal” ( below/equal to 102 cm and below/equal to 88 cm) and “High” (> 102 cm and > 88 cm) for males and females, respectively29.
Quality of life
Quality of life (QoL) of patients was assessed using a 21-item validated Audit of Diabetes-Dependent QoL (ADDQoL) questionnaire. The questionnaire comprises two general questions and 19-domain questions on the impact (ranging from − 3, the maximum negative impact of diabetes to + 1, the positive impact of diabetes) and importance (ranging from 3, very important to 0, not important at all) of diabetes. The 19-domain questions include: questions were leisure, working life, journeys, holidays, physical health, family life, friendship and social life, personal relationships, sex life, physical appearance, self-confidence, motivation, people’s reactions, feelings about the future, financial situation, living conditions, dependence on others, freedom to eat, and freedom to drink.
Each item was scored as Weighted impact score = impact rating (− 3 to + 1) * importance rating (0–3) = − 9 (maximum negative impact of diabetes) to + 3 (maximum positive impact of diabetes). The lower the WI score, the worse the aspect of life within the scope of a given domain. The average value of the weighted impact (AWI) score is also calculated for the whole scale. The AWI score is derived by dividing the sum of the weighted ratings by the number of applicable domains30.
Statistical analysis
Data was analyzed using frequencies and percentages for categorical variables. Means, standard deviation, and interquartile range were used for continuous variables. Pearson’s Chi-square and t-test were used to determine significant associations and differences between variables at p =< 0.05. All statistical analysis was carried out using statistical product and service solution version 25.